Introduction
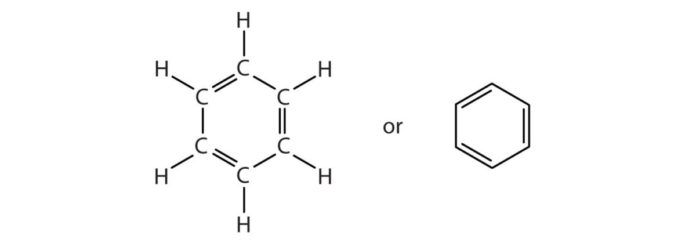
Benzene is the mother of all aromatic compound with chemical formula C6H6. Michael Faraday, an English scientist was the first one to discover Benzene in illuminating gas. The name benzene was given by German Chemist Mitscherich in 1833. The cyclic structure of this compound was discovered by German professor August Kekule in 1865. Volcanoes and forest fires produce benzene naturally.
Properties
- Immiscible in water
- Soluble in organic solvents
- Colorless liquid
- Density of 0.87g cm-3
- Boiling point 80.5°C and Melting point 5.5°C
- Resonating structures are formed
- Highly inflammable and burns with a sooty flame
Applications
- Used by the chemical industries for manufacture of lubricants, dyes, and rubbers.
- Used in the manufacture of detergents
- Degreasing of metals are done by this compound benzene
- Synthetic fibers like nylon contains the majority of benzene derivatives
- Chemical compounds like nitrobenzene, benzoic acid, cyclohexane, ethyl benzoate, etc are produced taking benzene as the raw product
- It is a major part of gasoline products
- It plays a vital role in the printing industries
Aromatic compounds are ring-shaped compounds that contain sigma bonds and delocalized pi electrons. In other words, these are also referred to as hydrocarbons as they contain majority hydrogen and carbon and hence the name hydrocarbons. There are unsaturated hydrocarbons present in these compounds and contain six-carbon ring structures called benzene ring.
Properties
- Aromaticity is the key characteristic in these aromatic rings
- The ratio of carbon to hydrogen is high in these molecules.
- Strong and sooty flame is produced on burning
Reactions by these aromatic hydrocarbons
● Substitution reactions:
The replacement of one of the substituents present on the benzene ring, basically a hydrogen atom is called substitution reaction.
The different types of these substitution reactions are:
(a) Nucleophilic aromatic substitution reaction
(b) Electrophilic aromatic substitution reaction
(c) Radical nucleophilic aromatic substitution reaction
● Coupling reactions
The two radicals present in the compounds are coupled together in the presence of a metal catalyst. During these reactions carbon to carbon, carbon to oxygen, and carbon to nitrogen bonds are formed.
● Hydrogenation reaction
The reduction reaction which leads to the addition of hydrogen to the molecule. Inorganic compounds, the compounds become more stable and saturated when the molecule or compound undergoes this reaction. Hydrogenation reactions reduce the number of double bonds and triple bonds, but in the case of dehydrogenation, it increases the number of double bonds and triple bonds.
Applications
- The hemoglobin present in our body is an aromatic hydrocarbon and helps in the transfer of oxygen to all parts of the body
- The chlorophyll present in plants helps in the process of photosynthesis
- The different acids like nucleic and amino acids present in the human body help in the proper functioning and synthesis of proteins
- These play a vital role in industries of synthetic fibers and petrochemical industries
- Medicinal chemistry takes a higher step in the production of drugs and medicines by using aromatic hydrocarbons
- Naphthalene, which is used as mothballs for domestic purpose is also an important hydrocarbon
- The vital compound 2,4,6 tri nitro toluene (TNT) is used in the production of explosives